Search Product
Structure Search
Search
Advantage Products
Location: Industrial Info
The Chinese University of Hong Kong Has Developed a Molecular Crowding Electrolyte For High-voltage Aqueous Batteries
Recently, Yijun Lu and others from the Chinese University of Hong Kong have developed a molecular crowding electrolyte with a wide voltage window, which is conducive to the realization of a safe, high energy density, sustainable development of lithium ion batteries. Related results were published in the recent "Nature Materials" journal.
In the research of water-based lithium-ion batteries, the use of high-salt-concentration water electrolytes, namely "water-in-salt electrolyte" (WiSE), is considered to be an effective method to extend the voltage window of water-based lithium-ion batteries, but this method is costly and causes People have paid attention to the toxicity of water-based batteries. In view of this, the researchers have inspired by "molecular crowding" and designed this new type of water-based electrolyte.
Molecular crowding is a common phenomenon in living cells where water activity is substantially suppressed by molecular crowding agents through altering the hydrogen-bonding structure.
The researchers used water-miscible polymer polyethylene glycol as a crowding agent to reduce the activity of water molecules. When the solvent contained a low content (2 mol kg-1) of bis(trifluoromethane)sulfonimide lithium salt (LiTFSi), it can completely inhibit the decomposition of water, thereby achieving a wider electrolyte working range (3.2 V)
.
The researchers assembled an aqueous full-cell Li4Ti5O12/LiMn2O4 based on this aqueous electrolyte, using LiMn2O4 as the positive electrode and Li4Ti5O12 as the negative electrode, and tested their reversible electrochemical behavior using CV. The results of on-line electrochemical mass spectrometry indicate that the molecularly crowded aqueous electrolyte avoids the decomposition of water in the L-LTO/LMO full battery. The full battery charge-discharge curve and electrochemical stability test show that the full battery can achieve an initial energy density of 110 Wh·kg-1 at a rate of 1C and maintain an energy density of 75 Wh·kg-1 after 300 cycles, and It can maintain high Coulomb efficiency during charging and discharging.
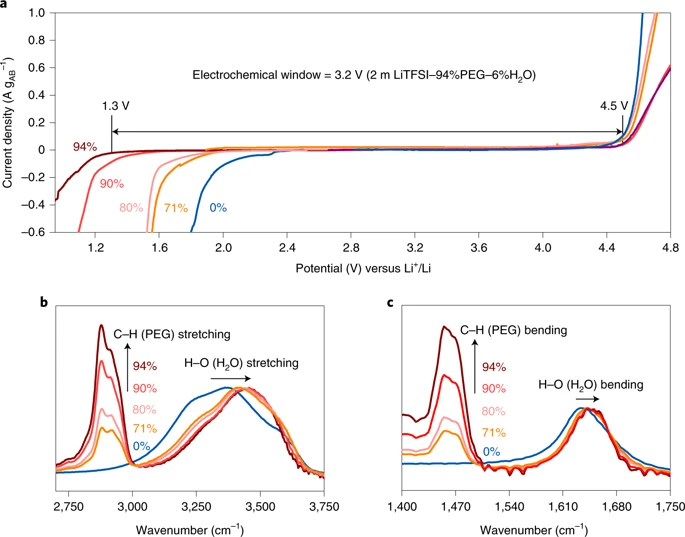
This research provides ideas for designing a low-cost, environmentally friendly, and wide-voltage window aqueous electrolyte.
References: Jing Xie, Zhuojian Liang, Yi-Chun Lu, Molecular crowding electrolytes for high-voltage aqueous batteries, Nature Materials, DOI: 10.1038/s41563-020-0667-y












