Search Product
Structure Search
Search
Advantage Products
Location: Thematic focus
What is graphene? Why is it widely favored?
As one of the emerging strategic materials, graphene has the characteristics of stable structure, high conductivity, high toughness and high strength. Graphene is a single layer of carbon atoms closely packed into a two-dimensional hexagonal honeycomb lattice structure. Each carbon atom is connected by sp2 hybridization, the angle between C-C is 120°, the bond length is about 0.142 nm, the bond energy is strong, and the structure is very stable. The following is a brief introduction to the preparation of graphene.
Preparation method for graphene
1. Mechanical peeling method
The mechanical stripping method is one of the earliest methods for preparing graphene. Novoselov used this method when it first discovered graphene. In the experiment, the graphite sheet was first peeled off from the graphite, and then the two sides of the graphite sheet were adhered to a special tape to separate the graphite sheets while tearing the tape. Continuously performing such a mechanical force stripping operation, the obtained graphite sheet becomes thinner and thinner, and finally a graphene composed of only one layer of carbon atoms is obtained, and the size of the graphene layer is d≥3 nm, about 100 μm long, and the naked eye visible. The method of the mechanical stripping method is easy to handle, but the prepared graphene has a limited size and cannot control the number of layers of graphene, and the yield is not high.
2. Epitaxial growth method
Berger et al. used a high temperature to heat a large area of single crystal SiC to grow graphene thereon, removing Si under ultra-vacuum or normal pressure to leave C, and then obtaining a thin layer of graphene having a similar area to the original SiC. In the process of studying epitaxial growth of graphene, it is found that there are many kinds of materials that can be used as graphene substrates, which are divided into non-metal substrates (including SiC, SiO2, GaAs, etc.) and metal substrates (including Cu, Ni, Co, Ru, Au, Ag, etc.). The Sprinkle and Heer research groups used a high-vacuum vacuum to heat up to 1000 °C to remove surface oxides and then heat the SiC surface to promote graphene growth. Emtse et al. used graphene grown on SiC surface under normal pressure, and the obtained graphene had an electron mobility of 2000 cm2V-1•S-1 at T=27K and 2700 cm2V-1•S-1 at room temperature. However, the graphene produced by the epitaxial growth method still cannot reach a uniform thickness, and the different substrate materials used may have different effects on the growth of graphene, and the graphene is not easily separated from the substrate material. Therefore, this preparation method still requires further experimentation and research.
3. Metal catalytic method
Metal catalysis refers to the method of directly forming graphene on a substrate by a solid or gaseous carbon source under a certain temperature, pressure and catalyst. Commonly used methods include chemical vapor deposition (CVD) and metal catalysis. Ren Wencai, a national (joint) laboratory of the Shenyang Institute of Materials Science, Institute of Metals, Chinese Academy of Sciences, used a noble metal platinum growth matrix to successfully prepare millimeter-scale hexagonal single crystal graphene by atmospheric pressure CVD with low concentration of methane and high concentration of hydrogen. Through the electrochemical gas intercalation bubbling method invented by the research group, the graphene film grown on the platinum can be transferred to any substrate without damage. The transferred graphene has a high quality and is transferred to a Si/SiO2 substrate to form a field effect transistor. The measurement shows that the single crystal graphene has a carrier mobility of 7100 cm2 V-1·S-1 at room temperature. The method is simple in operation, fast in speed, non-polluting, and suitable for transfer of noble metals such as ruthenium and osmium, and graphene grown on common metals such as copper and nickel. The metal matrix can be reused, and can be used as a low-cost, high-speed transfer method. It lays a material foundation for the practical application of graphene in high-performance nanoelectronic devices and transparent conductive films.
Now scientists have studied the mechanism of interaction between graphene and various substrates in epitaxial growth and metal catalysis, including atomic bond interaction and mechanism between substrate interface and graphene growth, and lattice matching, electron exchange and transfer; the effect of different morphologies and graphene atoms on the growth of graphene; the effect of the activity of the substrate material on the growth of graphene; the effect of the structure and shape of the substrate to the structure and band gap of graphene.
The application of Graphene
1. Application of graphene in battery materials
Carbon-based carriers are generally used as carriers for supporting precious metal catalytic materials in fuel cells. In the catalysts on the market today, the interaction between precious metal catalysis and carbon-based materials is weak, and precious metal particles are easily migrated, agglomerated and poisoned during use, lead to rapid decay of activity. The application characteristics of using graphene as a carbon carrier for noble metals in fuel cells are shown in Table 1.
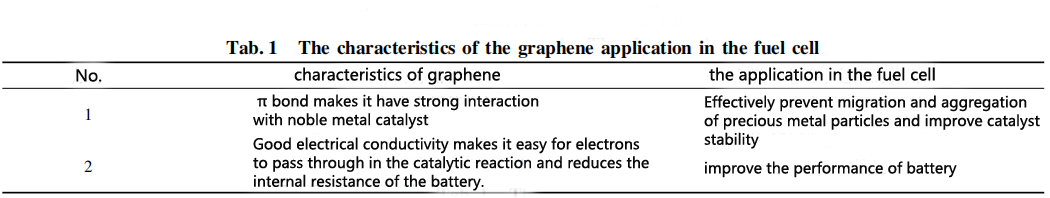
In order to obtain a composite in which graphene is effectively combined with a noble metal catalyst, the preparation method used is a simultaneous reduction and stepwise reduction to prepare a noble metal/graphene composite, and a graphene/precious metal composite prepared by in-situ template method. Synchronous and step-by-step reduction methods are to reduce graphite oxide to graphene. Both methods can successfully prepare graphene/precious metal (Pt, Pd) composites. Zhao et al. used the sacrificial template method to obtain Pd catalytic particles with uniform size and good dispersibility of graphene support. The research results indicate that the research goal of graphene in fuel cell anode catalysis should be to two sides: make Pt, Pd, WC and other catalytic particles uniformly dispersed on the surface of graphene, and Further reduce the amount of precious metals without changing the catalytic activity, so as to broaden the application prospect of graphene as a low-temperature fuel cell anode catalysis.
2. Application of graphene in the field of nanoelectronic devices
The chips currently used in computers are generally silicon-based, and there is a phenomenon of heat generation during the calculation. Therefore, the silicon substrate can perform only a certain number of operations per second at room temperature, and graphene has good thermal conductivity and electron mobility. The movement of electrons in it is almost unimpeded, and it runs much faster than a computer using silicon devices. Combining the characteristics and advantages of both silicon and graphene, such as good thermal conductivity, electron mobility, electrical conductivity, and large specific surface area, the study of silicon-doped graphene nanoribbons can broaden graphene nanometers. The belt is further applied in the field of nanoelectronic devices.
3. Application of graphene in supercapacitors
Supercapacitors are divided into double-layer capacitors, tantalum capacitors and asymmetric capacitors, which are a new type of energy storage device. Graphene materials are generally used in double-layer capacitors. The specific surface area, conductivity and pore size and distribution are several important factors for the storage of electrical materials in electric double layer capacitors.
Edited by Suzhou Yacoo Science Co., Ltd.












