产品搜索
结构搜索
全站搜索
当前位置: 行业资讯
清华大学研究团队发现锂硫电池调控的全新途径
锂硫电池是极具应用前景的电化学储能体系,近年来引起了研究人员的广泛关注。锂硫电池具有远高于现有锂离子电池的理论能量密度(2600 Whkg-1),因而受到了广泛的关注和研究。
锂硫电池的局限性
锂硫电池主要由正极、黏合剂、电解质、隔膜和负极组成。锂硫电池在理论上具有相当高的能量密度,但是其容量的衰减迅速,且锂硫电池存在硫正极电导率低、多硫化合物的“穿梭效应”、锂离子沉积以及在充放电过程中由于体积巨大变化产生的安全隐患等问题,束缚了它在实际中的运用。
锂硫电池中常用的醚类电解液,对于反应中间产物多硫化物的溶解度较低,导致电池中需要大量的电解液,限制了锂硫电池的实际能量密度。提高电解液的介电常数(ε)会显著增加多硫化物溶解度,有可能减小所需的电解液量。但此前的研究表明,在常用的高介电常数溶剂,如二甲基亚砜(DMSO)和二甲基乙酰胺(DMA)中,金属锂的稳定性较差。因此,这类电解液在从未实现在锂硫电池中的实际应用。
不同高介电常溶剂对锂硫电池性能的影响
近日,清华大学张强教授课题组比较了不同高介电常溶剂对锂硫电池性能的影响,发现基于四甲基脲(TMU)溶剂的高介电常数电解液,对金属锂负极具有较好的稳定性。一方面,相比于DMSO和DMA电解液,TMU电解液与高度活泼的金属锂兼容性更好,不会发生严重的溶剂分解反应;另一方面,TMU(ε≈24.5)电解液可以溶解数倍于醚类(DME,ε≈7.2)电解液的多硫化物,有助于减少电解液用量。研究者们注意到,高介电常数电解液中锂硫电池的反应路径与醚类电解液中显著不同:使用拉曼、紫外光谱等手段,他们证实了TMU电解液中存在S3•−自由基负离子;通过比较不同电解液的锂硫电池在恒定电压或电流下放电行为的区别,他们发现较高的多硫化物溶解度有利于提高正极活性物质的利用率,促进硫化锂的三维沉积,减缓硫化锂对正极导电骨架的钝化。将这种电解液用于软包电池,在3:1的电解液/硫质量比下,可以实现324 Wh kg-1的能量密度。
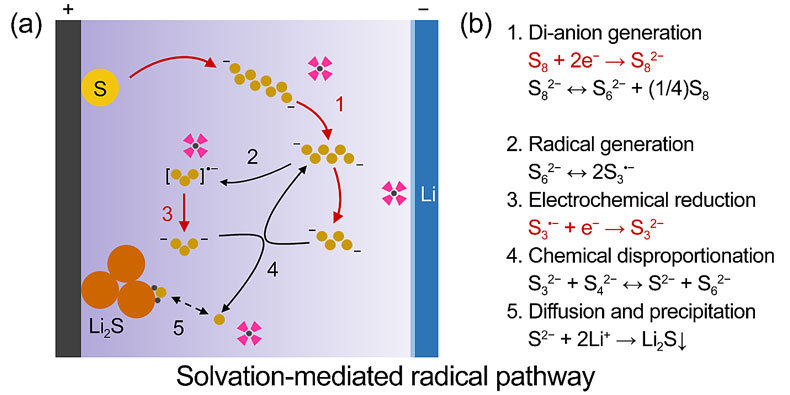
TMU为锂硫电池电解液溶剂提供新思路
基于负极稳定的自由基反应路径,TMU被选为锂硫电池电解液溶剂,实现了更高的活性物质利用率。相比于传统醚类电解液,TMU可以溶解更多的多硫化物,在其中硫化锂倾向于三维沉积,活性物质硫的利用率得以提高。同时TMU在金属锂负极的好于其它高介电常数溶剂,抑制了锂盐的分解,生成以无机成分为主的SEI。TMU电解液首次在锂硫电池中用高介电常数溶剂实现了稳定循环,并且在贫电解液的软包电池中获得了91%的硫利用率和324 Wh kg-1的能量密度,为新型电解液的研发提供了新的思路。该工作也为未来锂硫电池的发展提供了一条基于电解液调控的全新途径。
本文由苏州亚科科技股份有限公司编辑












